This is a technical post by Harry Mercer on Indigo dyeing. It is the third part of the article in series. The first part can be seen by clicking here and the second one here
Part 3 of 4: Monitoring of the Indigo Dye baths
As discussed in Part 2 of this series, most of the control of Indigo dyeing must be managed before the dye and chemicals reach the dye boxes in the machine. In order to measure the effectiveness of control measures of the dye and chemical mixes, it is necessary to measure concentrations of Indigo, reducing agent and alkali in the Indigo dye boxes. If the methods of measuring these concentrations in the dye boxes are accurate and reliable, then those measurements will be able to predict the Indigo color of the yarn and the colorfastness of the denim in the garment laundry as well as providing a benchmark of dyeing management capability. By analyzing the data for each dyeing method with simple statistical tools such as Standard Deviation, the level of control for each color, for each dye lot and each group of workers can be measured and improved. In this way, continual progress can be made towards perfect denim color which has been achieved in a few companies. This wins business with jeans producers that pay the highest prices.
In 1992, I published an article, “Quality Assurance Methods for the Continuous Dyeing of Cotton Yarn with Indigo” that was the most advanced treatment of the subject. After years of research into test methods for Indigo dyeing, several conclusions could be made:
- 1) the most commonly-used methods of measurement, pH and millivolts were unreliable and could not be correlated with the dyeing results. They gained popularity because of simplicity and because that the results were consistent- the color changed, but the pH and millivolts were consistent. Dye managers used these numbers to claim that they had good dyeing control when customers complained about color variation.
- 2) Statistical Analysis comparing test results with the actual fabric color and wash-down demonstrated that the glass plate, vat-ometer and a special alkali titration correlated perfectly with instrumental color measurements of denim(L*a*b* or L*c*h*) and were the only reliable test methods for correcting Indigo dyeing problems.
The primary cause of Indigo variation is changes in the reduction potential of the dyebath which consists of a combination of reduced Indigo and free sodium hydrosulfite. The total hydrosulfite in the Indigo bath is divided between the hydrosulfite that is consumed for reduction of the indigo dye molecule and free hydrosulfite that is not consumed in dye reduction. Where the dye circulation is adequate and the dye feeding concentrations are consistent, the grams per liter of reduced Indigo and the alkali levels are consistent. The free hydrosulfite is unstable and requires the most effort in control for Indigo dyeing. As the concentration of free hydrosulfite increases, the Indigo shade becomes greener and more wash-fast; as the free hydrosulfite decreases, the indigo appears less green or redder and loses color faster after washing. If the free hydrosulfite changes by 0.3 grams per liter in the indigo bath, a different indigo shade results. Many denim companies have 10-15 shades per dye lot where a true color difference of 0.2 Delta is used as a measure.
In the above-mentioned article that I published, I mentioned a number of possibilities for measuring reduced Indigo and free hydrosulfite in Indigo dye boxes. One was the permanganate method that uses a 2-endpoint, potentiometric reduction-oxidation titration.This method has come into common use in many companies, but is not reliable for measuring the Indigo dye. The method has been in use for over a hundred years and I found it useful in conducting research into Indigo dyeing in order to develop Indigo formulations in the laboratory. This method was occasionally useful at the Indigo machine when developing new Indigo colors, but only with manual titration. Automatic titrators usually proceed too quickly and produce erroneous endpoint identifications. Also, the sample sizes used are too small to be representative of the average Indigo and hydrosulfite concentrations for all of the dye boxes. In addition, the test is too slow for quick response in dyeing and laboratory personnel often are not aware of maintenance and calibration requirements.
The vat-ometer, on the other hand, offers simplicity, speed and reliability. This device was invented about 150 years ago and research into Indigo dyeing has demonstrated that the vat-ometer results are consistent and are predictive of actual color variations in Indigo dyeing. The vat-ometer consists of a rounded glass flask and a measuring tube. Water is added to the vat-omter, then dyebath is entered, the vat-ometer is sealed to prevent entry of additional air and then the mixture is shaken for about 1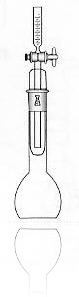
As mentioned previously, the practical number is the average hydrosulfite for all of the dye boxes. As a rule the hydrosulfite concentrations in indigo dye boxes will vary from box-to-box, from top-to-bottom and from side-to-side. In order to know the machine average, at least 2 dye boxes should be sampled. For example, on a 6-box Indigo machine, normally an average of the 2nd and 5th box will equal the machine average.
The vat-ometer offers the advantages of larger sample sizes for better accuracy faster results. The vat-ometer can be constructed in the laboratory to use dye samples as large as 100 cc’s, however the portable vat-ometer which yuses a 10 cc sample has long been proven to be accurate enough for Indigo dyeing production eg the one from Tudorscientific here.



